A guide to smashing A-level Chemistry
How to give yourself the best chance of securing that A*
CHEMISTRY
Louisa
9/13/20239 min read


A-level Chemistry - you either love it or you hate it
Difficult-to-understand concepts are the hallmark of A-Level Chemistry. These concepts (which seem to get ever harder as you move through the content), plus losing marks to the highly specific mark schemes, can be really discouraging when picking up the subject in year 12, or moving from year 12 to year 13 content. Common struggles also include calculations, understanding organic chemistry mechanisms and inorganic subjects - commonly more memorisation-heavy. It is often the case that you can spend hours revising the content, but still not see your hard work reflected on topic tests and mocks. This guide compiles advice from our tutors who have sat A-level Chemistry themselves to gain top scores, as well as tutored the subject for several years. We know what works and what doesn't and we'd like to share that with you.
The process
Mastery of Chemistry involves many different aspects. This is split into understanding concepts as they are taught to you, regularly revisiting concepts and thorough practice of exam technique. We can also include some more chemistry-specific skills such as practising calculations, drawing organic mechanisms and understanding practical technique.
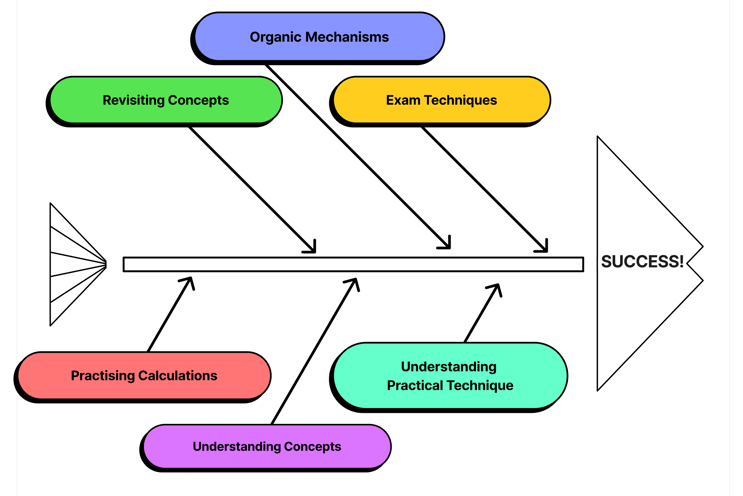
Drawing Organic Mechanisms
Understanding the rules behind how to draw mechanisms in organic chemistry will change the game for you in how to approach these questions. Although there are obviously always different things to remember for electrophilic addition vs nucleophilic substitution there are rules which underpin the way we always draw mechanisms.
Key rules
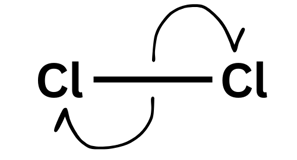
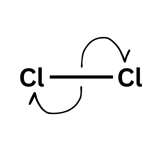
Arrows go:
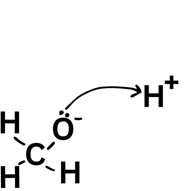
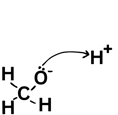
From negative to positive - when they go from a species with a negative charge on lone pair the arrow must always come from that lone pair.
OR
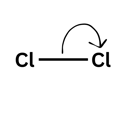
From the middle of a bond out (showing the bond is breaking)
Bonds breaking
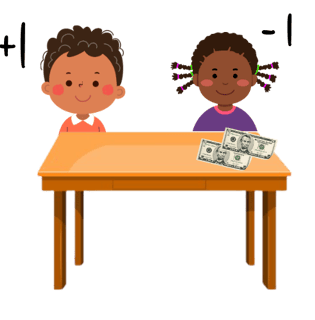
Think about each molecule in a bond depositing one electron into that bond - like sitting at a table with your friend and each putting down a £5 note onto the table.
To summarise
Double-headed arrow = two electrons moving
Single-headed arrow = one electron moving
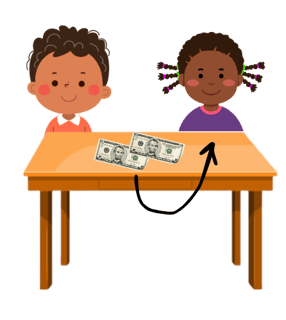
A double-headed arrow shows two electrons moving - like your friend picking up both £5 notes. You would have lost £5 and they would have gained £5 (of the £10 they picked up £5 was theirs already and they gained £5). For the bond breaking that means that one molecule gains an electron and one loses an electron leading to a positive and negative ion forming.
A single-headed arrow is one electron moving. Imagine you and your friend both pick up your original £5 off the table. You would both leave with what you started with: £5. For molecules, this leads to the formation of a radical - a species with an unpaired electron. These are very reactive species which will attack other molecules in order to try and pair up its electron.






Remember that if a molecule gains an electron it is gaining a negative charge and therefore becomes -1.



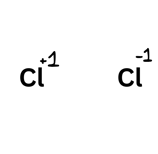
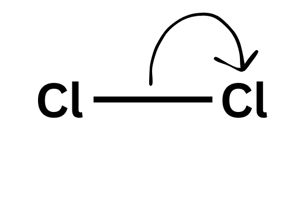
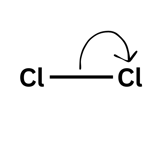








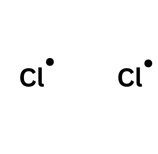
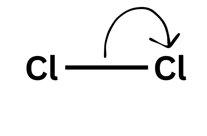
Practising Calculations
To succeed in A-Level Chemistry you must prioritise understanding calculations. That means, right from the get-go in Year 12, keeping up to date with calculation knowledge. Topics such as Amount of Substance, Hess' law and Bond enthalpies are relatively simple in concept, once you get your head around them. The most difficult aspect of calculations is the application. Examiners love to throw confusing tricks into simple calculations. This means you can feel confident with the concept of a calculation, and yet still lose a lot of marks. For example, Ca(OH)2 has a 2:1 ratio in the moles of OH- compared to the moles of Ca(OH)2 - a common trick in titration questions. Flashcards are not normally useful for these calculations. So for spaced repetition (repeating things often for short periods of time to move it into your long-term memory), you should do regular practice at set intervals. You should attempt every type of question roughly every two weeks for 15-20 minutes. This won't be a massive chunk out of your day, but will make a significant difference in your A-Level performance. I recommend Physics and Maths tutors as an excellent resource to provide lots of free past-paper calculation questions separated by the exam board, to practice. It's worth noting that a lot of exam boards have overlap in questions, particularly calculation ones. So for, example, with AQA and OCR it would be totally fine to do practice calculation questions from either exam board.
Practical Technique
Visualising is key to understanding practical technique. We recommend finding the specific practical you're trying to study on youtube and watching experts carry it out. This should hopefully translate into your mind a better idea about the practical than reading instructions. The best case scenario would be to ideally do the experiment at least once in class, but different schools approach the practical endorsement differently, so this may not always be possible.
To check that you've mastered a specific practical there's a couple things you can do. Try explaining the experiment to a friend (ideally someone who studies A-level chemistry) as if they have never heard of it before. Use key words when talking about the apparatus and the techniques e.g the titre and the conical flask. As well as this, have a go at some practical style exam questions. A lot of these questions will be 6 markers, where you are expected to discuss if not all then most of a practical technique. The mark schemes of these questions are remarkably valuable, as they are going to tell you exactly what the examiner is looking for. Redo these questions several time until you can confidently hit each point of the mark scheme.
For the remainder of those pesky practical questions - read on to our exam practice section to find out exactly how to boost your confidence in them.
Understanding concepts
A very important part of the process is making sure things make sense when they're being taught to you.
Some days, you can passively sit in lesson and let the content go straight over your head. I speak from experience when I say its also very easy to lose focus for a couple minutes in a lesson, then be completely out of sync for the rest. In these instances, its best to ensure you go over the content again and make sure it sits well in your head. This definitely isn't always easy, and difficult concepts will not always go in the first time. You have to be persistent, and access different resources available to you. This can be notes online, textbooks and revision guides, YouTube videos and definitely tutoring. Our tutors are always happy to spend as much time with you as you need, making sure you understand concepts at the right level.
Revisiting concepts
By the time you've finished going through the (huge) specification, topics that you covered in year 12 or at the start of year 13 may be feeling a bit alien to you. This isn't how you want to be feeling around exam season, which is why it is essential that you cover topics regularly to keep them fresh.
We recommend the use of Anki flashcards for this. This is an app you can install on your computer that helps you make flashcards and even use pre-existing decks that have been made by other students. Where Anki excels over websites like Quizlet and physical flashcards is the scheduling. The app learns what cards you are struggling with and schedules them for you to study more frequently until you are feeling more confident. Say, for example, the shapes of molecules just won't go in - Anki will schedule these cards to be studied frequently, until they make sense.
Ask one of our tutors to give you a quick demo on how to set up and use Anki. One of the only caveats to this amazing app is that it may be a little daunting to get started with, but once you get going it becomes very easy. Anki is the way all of our tutors are currently preparing for (and excelling in) medical school exams!
Regardless of whether you end up using Anki or not, make sure to revisit the content several times. Print off a copy of the specification and highlight the content you have covered and plan to revisit. For example, you could highlight a section in amber if it is something you have covered once or twice, and change that to green when you have gone over it sufficient times that you feel an expert on it. Red can be used for topics that you have not yet covered. You should note - most of your specifications will be amber and red until close to the exams! Don't be hasty in telling yourself you're an expert on something!
Mastering exam technique
As an A-level chemistry student, you're probably all too familiar with the feeling of thinking you really understand a topic, only to be extremely humbled by exam questions. The exams are usually structured in such a way that the majority of marks are not purely from recall, but rather from application of knowledge. The way to become an expert on these questions is not all that obscure: practice! The more exam questions you do, the better you get at scoring those top marks. We recommend that you also study mark schemes. There's often a lot to gain from looking at the columns there, to find out what key phrases examiners are looking for and what phrases are not awarded any marks.
In fact, a technique some of our tutors have used and recommended to great success is making flashcards of difficult exam questions. Say there is a weird five-marker that you have never seen before and scored zero or only one mark out of the five, turn it into a flashcard with the question on the front and the mark scheme on the back. Over time you should build a collection of these cards (on Anki or otherwise), that you are reviewing regularly. The point of this is that by the time your exams come around you have committed to memory a lot of difficult exam questions. Exam boards like to re-use very similar questions with minute changes, and this means that a question that might have left you confused before should be straightforward when encountered in the exam.
When answering questions, whether in your own timed practice or during mocks and even the real exam, we think you should always answer in bullet points. From 2 to 6 markers, bullet points show that you have confidence in your answer and make it easier for the examiner to see where your marks are coming from. Easier said than done though, as there's always the temptation to start blurting whatever you think is remotely relevant whenever you're not sure what the question is answering. Hopefully, by the time of the exam, you will have done lots of practice questions and seen many mark schemes, so you should know what specific exam questions are looking for.
Finally, for calculations, the best thing to do is to practice. The more applications and different varieties of questions you do, the better prepared for the exam you are. Make a note of the little tricks which get you - for example forgetting the ratio of moles is 1:2 in a titration question or not realising the formula of Calcium hydroxide is Ca(OH)2 rather than CaOH. If you fall for all these tricks in practice you won't fall for them in the exam or an important mock!
Thank you for reading this article! If you have any suggestions on how we might improve, or any questions, please get in contact!


